Overview of FDA Regulations on Human Cell and Tissue Based Products: 351 vs. 361 Classification | Stem Cell and Exosome Therapy USA & International

Evaluating the FDA regenerative medicine framework: opportunities for stakeholders | Regenerative Medicine

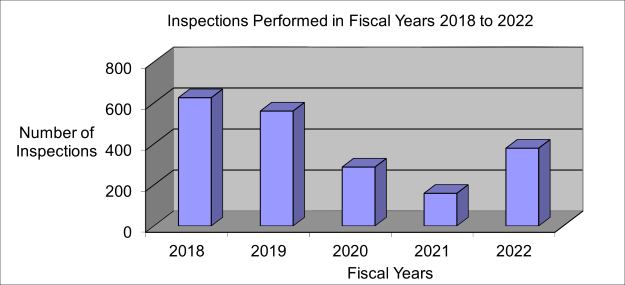
PPT – HCT/P Contamination Prevention and Biologic Product Sterility Regulations Applicable to PBSCs PowerPoint presentation | free to view - id: 22e501-MGU3O
Instructions for Using the Electronic Human Cell and Tissue Establishment Registration System (eHCTERS)
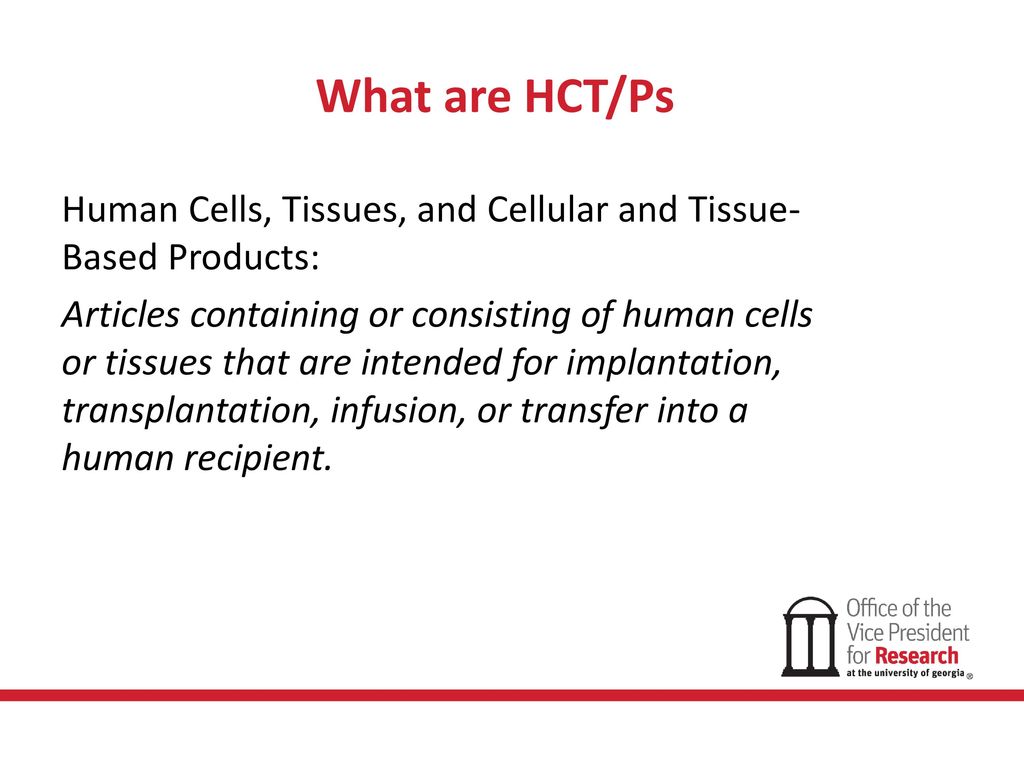
Introduction to Human Cellular and Tissue Based Products (HCT/P), Cell Therapy and Gene Therapy - Food and Drug Law Institute (FDLI)

FDA Issues First Untitled Letter of the Year to HCT/P Manufacturer | Sheppard Mullin Richter & Hampton LLP - JDSupra
Minimal Manipulation of Human Cells, Tissues, and Cellular and Tissue-Based Products Draft Guidance for Industry and Food and Dr